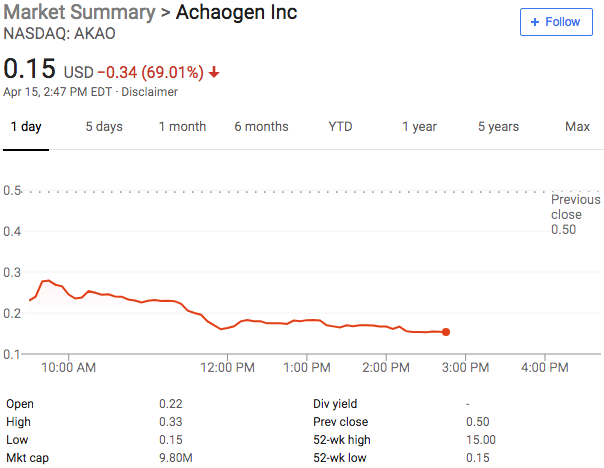
New Chapter 11 Bankruptcy Filing - Achaogen Inc.
Achaogen Inc.
April 15, 2019
Biopharma is where it’s at!!
San Francisco-based Achaogen Inc. ($AKAO) is the latest in a slate of biopharma debtors who have found their way into bankruptcy court — here, the District of Delaware. Achaogen is focused on “the development and commercialization of innovative antibiotic treatments against multi-drug resistant gram-negative infections.” To date, its operations have been centered around the discovery, development and commercialization of products, making it as far as clinical trials in certain instances. As if inspired by the fact that its filing came on the heels of the much-anticipated Game of Thrones (final) Season 8 premiere, the company colorfully notes its primary purpose:
Achaogen designed its lead product, ZEMDRI® (plazomicin), to fight what the Center for Disease Control (“CDC”) calls a “nightmare bacteria” and has listed as the highest category threat of “urgent.” ZEMDRI can be used to treat patients who have limited or no alternative treatment options from infections with these nightmare bacteria. Even with its current financial situation, Achaogen continues to commercialize ZEMDRI, in part because Achaogen believes that ZEMDRI can save lives for patients who may literally have no alternative.
Nightmare bacteria!! Sheesh that’s chilling.
Even more chilling is the company’s discussion of gram-negative bacteria — found “everywhere, in virtually all environments on Earth that support life.” These bacteria are becoming increasingly resistant to common antibiotics. Achaogen calls this “a global crisis…we take for granted.” The company’s core (patented) product, ZEMDRI, is designed to “retain its effectiveness in killing these more resistant bacteria.” While ZEMDRI received FDA approval for IV-treatment of patients with complicated urinary tract infections in July 2018, the FDA rejected ZEMDRI for treatment of patients with bloodstream infections, citing a lack of substantial evidence of effectiveness.
What does the company have going for it? Again, as of July 2018, it has a commercially viable product in the United States. It also has global commercialization rights. And patent protect in the US through approximately 2031 or 2032. It sells to either specialty distributors or physician-owned infusion centers. It has agreements with Hovione Limited and Pfizer for the manufacturing of its product. Finally, it has another product in development, C-Scape, which is an oral antibiotic for treatment of patients suffering from urinary tract infections caused by a particular bacteria.
So, what’s the issue? As PETITION readers have come to learn, the development and manufacture of biopharma products is a time and capital intensive process. Indeed, the company has an accumulated deficit of $559.4mm as of December 31, 2018. This bit is especially puzzling given the company’s position that the world confronts a “global crisis”:
In the past year, there has been a dramatic downturn in the availability of financing from both the debt and equity markets for companies in the anti-infective field, based in part on the withdrawal from the space by certain large pharmaceutical companies. For example, Novartis recently announced that it is shutting down its antibacterial and antiviral research, which was followed by similar moves from Eli Lilly, Bristol-Myers Squibb and AstraZeneca.3 Allergan has also recently announced its intention to divest its anti-infective business, consisting of three commercialized products. This “big pharma flight” from antiinfective research, development and commercialization has created significant challenges for early-stage biotech companies seeking to develop and commercialize novel and much needed drugs in this sector, as opportunities for partnerships, joint R&D relationships, and merger/acquisition transactions have diminished. This sector-wide trend has negatively affected not just Achaogen but many of its competitors. Achaogen, however, has been especially impacted because it has reached the point in its life cycle where it needs substantial capital infusion to drive commercialization of its recently FDA approved drug, ZEMDRI.
Look: we don’t take everything debtors say as gospel. After all, first day pleadings are an opportunity to frame the story and set the tone of a case. But if the company is right about what it’s saying and nobody appears to give two sh*ts, well, color us a wee bit concerned. Why isn’t anybody talking about this?
Anyway, in February 2018, the company entered into a loan and security agreement with Silicon Valley Bank for $50mm. The original agreement provided SVB with a security interest in virtually all of the company’s assets — including proceeds of intellectual property — but not a security interest in the IP itself. $15mm remains outstanding under the loan. In November 2018, the company retained Evercore Group LLP to run a strategic sale process but no viable purchaser emerged. It’s not worth saving the world unless you can make some dinero, we suppose.
After engaging in various liquidity maximization efforts (including job cuts), fundraising initiatives (including an insufficient equity raise), and licensing discussions with entities abroad, the company ultimately decided that nothing would generate enough liquidity for the company to avoid chapter 11. The company notes, “although Achaogen’s out-of-court sale process did not yield any acceptable bids, many parties had expressed interest in bidding at any potential 363 auction sale, where it could pursue the Assets free and clear of existing liabilities.” The company, therefore, filed for chapter 11 to pursue a new sale process; it has no stalking horse bidder teed up. To market its assets, the company has replaced Evercore with Cassel Salpeter & Co. LLC.
In support of the bankruptcy case, SVB committed to provide the company with a $25mm DIP credit facility of which $10mm is new money and $15mm is a roll-up of the aforementioned pre-petition debt. In exchange, SVB now gets a security interest in the company’s IP.
The company’s unsecured debt is comprised of lease obligations, minimum purchase requirements under its manufacturing contract, a success fee tied to the company’s FDA approval, and $18.7mm of trade debt. New Enterprise Associates Inc., a reputed Silicon Valley venture capital firm, is the company’s largest equity holder with approximately 10.76% of the company’s shares. Prior to its 2014 IPO, the company had raised $152.1mm starting with its Series A round in August 2004: it IPO’d at a valuation of $200.4mm, having issued 6.9mm shares at $12/share to the public. It’s equity is likely worth f*ck all. Well, not exactly: we suppose this isn’t ENTIRELY “f*ck all”:
But it’s pretty darn close. Now the issue is what price the IP will fetch in a bankruptcy sale process. It will have to be tens of millions of dollars for NEA to have any sort of recovery.
Jurisdiction: D. of Delaware (Judge Shannon)
Capital Structure: $15mm secured debt (Silicon Valley Bank)
Professionals:
Legal: Hogan Lovells US LLP (Erin Brady, Richard Wynne, Christopher Bryant, John Beck) & (local) Morris Nichols Arsht & Tunnell LLP (Derek Abbott, Andrew Remming, Matthew Talmo, Paige Topper)
Financial Advisor: Meru LLC
Investment Banker: Cassel Salpeter & Co., LLC
Claims Agent: KCC (*click on the link above for free docket access)
Other Professionals:
Prepetition & DIP Lender ($25mm): Silicon Valley Bank
Legal: Morrison & Foerster LLP ( Alexander Rheaume, Todd Goren, Benjamin Butterfield, David Ephraim) & (local) Ashby & Geddes PA (Gregory Taylor, Stacy Newman)
Official Committee of Unsecured Creditors (Hovione Limited, EsteveQuimica SA, Solar Capital Ltd.,. Crystal BioScience, World Courier)